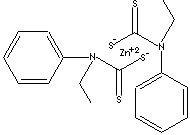
ZINC ETHYLPHENYLDITHIOCARBAMATE
PRODUCT IDENTIFICATION
H.S. CODE
TOXICITY
Accelerator EFK; Bis(ethylphenylcarbamodithioato-S,S')zinc; Fenyl-ethyldithiokarbaminan zinfonaty; Bis(N-ethyldithiocarbanilato)zinc; Hermat FEDK; Zinc ethylphenylthiocarbamate;
SMILES
CLASSIFICATION
Dithiocarbamate, Vulcanization accelerator
PHYSICAL AND CHEMICAL PROPERTIES
white to off white powder
MELTING POINT
SOLUBILITY IN WATER
Insoluble
REFRACTIVE INDEX
Health: 1, Flammability: 1, Reactivity: 0
GENERAL DESCRIPTION & APPLICATIONS
Dithiocarbamate is the analog of carbamate in which both oxygen atoms are replaced by sulfur atoms. carbamates are inter-related structurally and often are interconverted chemically. Dithiocarbonate is the thio analog of carbonate. Thiocarbonates contain sulphur in place of oxygen. Xanthate is one of important dithiocarbamate. Dithiocarbamates and dithiocarbonates readily react with many metal salts and are used as ligands when metal salts are added to it. Coordination complexes of zinc metal with anionic ligands dithiocarbamates and dithiocarbonates are used as secondary accelerators for rubber vulcanization.
ZEPC is a fast primary or secondary rubber accelerator. It is also used as an antioxidant in rubber-based adhesive systems.
APPEARANCE
white to off white powder
ASSAY
98.0% min
MELTING POINT
200 C (Initial), 215 C (Final)
SIEVE ANALYSIS
0.5% max (+ 63 µm)
There are some types of rubber accelerators. They are used in combination with each other in accordance with vulcanizing and/or acid-base conditions. Some examples classified by chemical structure are as below;
- Thiazole
- 2-Mercaptobenzothiazole (CAS #: 149-30-4)
- Dibenzothiazole disulfide (CAS #: 120-78-5)
- 2-Mercaptobenzothiazole Zinc salt (CAS #: 155-04-4)
- Sulphenamide
- N-Cyclohexyl-2-benzothiazole sulfenamide (CAS #: 95-33-0)
- N-Oxydienthylene-2-benzothiazole sulfenamide (CAS #: 102-77-2)
- N-tert-butyl-2-benzothiazyl sulfenamide (CAS #: 95-31-8)
- Guanidine
- Diphenyl guanidine (CAS #: 102-06-7)
- Di-o-tolylguanidine (CAS #: 97-39-2)
- Thiuram
- Tetramethyl thiuram disulfide (CAS #: 137-26-8)
- Tetraethyl thiuram disulfide (CAS #: 97-77-8)
- Tetramethyl thiuram monosulfide (CAS #: 97-74-5)
- Isobutyl thiuram disulfide (CAS #: 3064-73-1)
- Tetrabenzylthiuram disulfide (CAS #: 10591-85-2)
- Dipentamethylene thiuramtetrasulfide (CAS #: 120-54-7)
- Dithiocarbamate
- Zinc dimethyl dithiocarbamate (CAS #: 137-30-4)
- Zinc diethyl dithiocarbamate (CAS #: 14324-55-1)
- Zinc dibutyl dithiocarbamate (CAS #: 136-23-2)
- Zinc N-ethyl-dithiocarbamate (CAS #: 14634-93-6)
- Zinc dibenzyl dithiocarbamate (CAS #: 14726-36-4)
- Copper dimethyl dithiocarbamate (CAS #: 137-29-1)
- Thiourea
- Ethylene thiourea (CAS #: 96-45-7)
- N,N'-Diethylthiourea (CAS #: 105-55-5)
- N-N'-Diphenylthiourea (CAS #: 102-08-9)